A new standard, ASTM C1866, specifies ground glass for use in concrete. It’s part of a trend to promote sustainability by making use of marginal and recycled materials in concrete. ASTM C1709 specifies a protocol for evaluating marginal fly ashes, slags, and silica fumes that don’t meet their respective ASTM standards. These marginal materials may still be acceptable in certain applications. Following the evaluation protocol helps ensure acceptable performance in concrete.
The focus of ASTM C1866 is on the use of recycled glass in concrete. The glass may be from containers, plate glass, or E-glass (that is, glass from glass-fiber reinforcement). It promotes sustainability by specifying how post-consumer waste glass can be safely used in concrete. Use of recycled materials in one way to meet the requirements of CALGreen, or to achieve net zero concrete.
What is glass?
The term “glass” refers to the microstructure of a class of materials, regardless of chemical composition. For inorganic materials, the state of lowest energy is crystalline—an ordered array of atoms. But when molten material cools quickly enough, there isn’t time for the atoms to form an ordered array. They solidify in an amorphous arrangement we call glass. This can occur in nature, for example when lava flows into the sea. It can also occur in manufacturing, as when we quench molten material rather than let it cool naturally.
For a given chemical composition, the difference between the glassy and crystalline states is reactivity. That goes back to the energy state of the microstructure. The higher the energy state, the more reactive the material—it wants to go back to its lowest energy state. That is, a glass is more reactive than its crystalline counterpart.
Glass in concrete
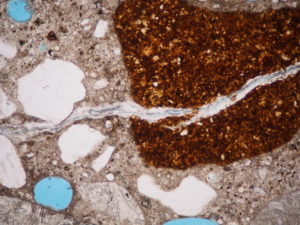
For glass in concrete, the reaction we’re most concerned about is alkali-silica reaction. Alkalis from the cement react with amorphous silica, generating a gel that expands as it imbibes water. Strangely, this reaction can be either beneficial or highly destructive—it’s all in the timing. If it takes place after the concrete has hardened and gained strength, it can cause destructive cracking, as in the photo.
“Classic” alkali-silica reaction occurs with aggregates such as opaline shale, siliceous limestone, or obsidian, which comprise glassy forms of silica. However, artificial glass will also react in concrete. When we use glass fiber as a reinforcing material, we have to make sure it isn’t susceptible to alkali-silica reaction.
Sometimes people want to use broken glass in concrete. For example, an artist might want to make a mosaic using glass fragments of different colors. Or someone may think glass aggregates in terrazzo or concrete would be a good way to make use of post-consumer waste glass. However, this kind of application is likely to end in tears—the glass will react, generate gel, and expand, causing the three-armed cracks and eventual disintegration characteristic of alkali-silica reaction.
Mitigating alkali-silica reaction
In many parts of the country, it’s either impossible or very expensive to obtain aggregates that won’t react like this. Here in Minnesota, we can specify granite (crystalline silica) aggregates at a premium price. In New Mexico, all the local aggregates are highly reactive. What do you do then?
It’s all in the timing. If you have a reactive aggregate, you need to provide a source of silica that will react with the alkalis before the aggregate does. Supplementary cementitious materials such as fly ash, silica fume, and slag cement are all effective mitigation measures. You can use 1567 to test combinations of materials with your aggregate to make sure the expansions are within acceptable limits.
ASTM C1866
While you wouldn’t want reactive glass as aggregate in concrete, finely ground glass is another story. That’s where ASTM C1866 comes in.
ASTM C1866 specifies two classifications of glass:
- Type GS (ground soda-lime-silica glass), typically from container- and plate glass, with equivalent alkali content of 10 to 15%
- Type GE, typically from ground glass fiber, with equivalent alkali content of 0 to 1%.
Chemical requirements include minimum percentages of silica and maximum percentages of alumina and lime (CaO). An optional requirement, which the purchaser must request, calls for a minimum amorphous content of 95%.
Physical requirements include the 7- and 28-day strength activity index. In addition, the water requirement and relative density must be reported. At the request of the purchaser, optional physical requirements include specific surface and effectiveness in contributing to sulfate resistance. Sulfate resistance is important in the Great Plains and the western United States, where sulfate-bearing soils are prevalent.
Testing for mitigation of alkali-silica-related expansion
If you want to use ground glass to mitigate alkali-silica reaction, you can test combinations of Type GE ground glass just as you would fly ash, slag cement, or silica fume, with ASTM C1567. However, if you want to use the high-alkali Type GS, you need to use a different test, ASTM C1293. In this application you’ll want to use it in combination with other supplementary cementitious materials.
It’s important to note that ASTM C1567, a test of crushed aggregate in mortar, takes a few weeks to complete. On the other hand, ASTM C1293 tests coarse aggregate in concrete. Testing the effectiveness of mitigation measures using this method takes two years.
